Understanding the Chemistry Behind Softer, Cleaner Water for Your Home
Key Takeaways
- Protect your plumbing and appliances by understanding how ion exchange replaces damaging minerals with harmless sodium or potassium ions.
- Master the mechanics of resin beads and how they act like magnets to trap scale-causing contaminants.
- Keep your system efficient with our simple guide to regeneration cycles and maintenance schedules.
Dealing with crusty white scale on your faucets, spots on your glassware, and dry, itchy skin can be incredibly frustrating for any homeowner. These are the classic signs of hard water, and while the solution often involves an “ion exchange” system, the term itself can sound like a daunting chemistry experiment. The good news is that the process is actually quite straightforward and incredibly effective at protecting your plumbing and appliances. We’re here to demystify the science behind this technology so you can understand exactly how it improves your home’s water quality.
What Is an Ion Exchange Water System?

At its core, an ion exchange water system is a physical and chemical process used to remove specific impurities from your water supply. You will most commonly find this technology inside a standard home water softener. These systems typically consist of three main components: a mineral tank where the softening happens, a brine tank that holds the salt, and a control valve that manages the flow. Its primary goal is “softening” or “demineralization,” which involves removing dissolved solids like calcium and magnesium that make water hard. Unlike simple mechanical filters that trap debris in a mesh, an ion exchange water filter works on a molecular level to swap unwanted minerals for harmless ones. This water treatment method is the industry standard for combating hardness because it physically removes the minerals rather than just preventing them from sticking to pipes.
The Science Simplified: How the Ion Exchange Process Works
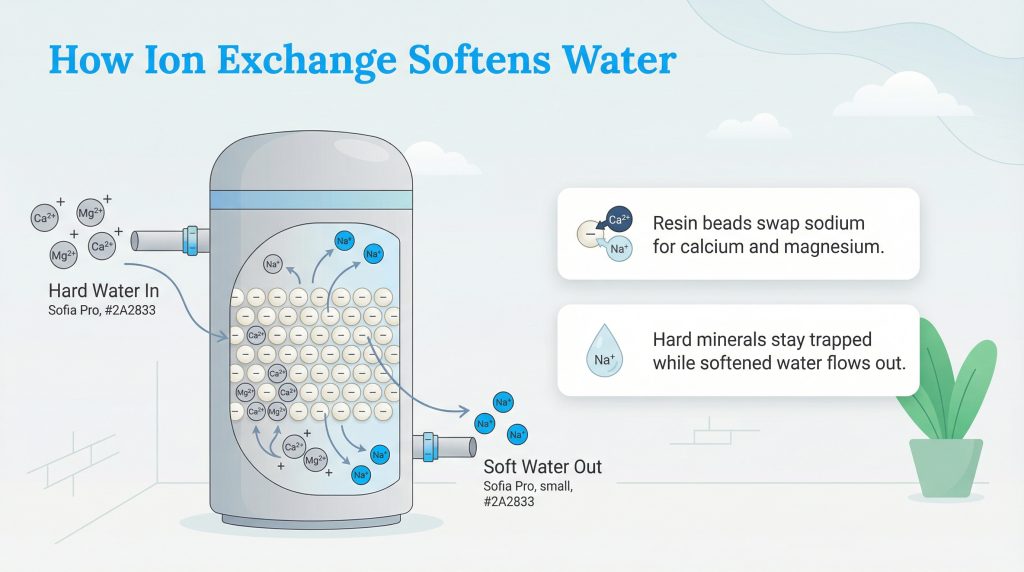
To understand how ion exchange water systems work, it helps to visualize a simple magnet. Inside the tank of your water softener, there are thousands of tiny plastic spheres called ion exchange resin beads. These beads are negatively charged. Because opposites attract, they hold onto positively charged sodium ions. When hard water flows into the tank, it brings along calcium and magnesium minerals, which have a stronger positive charge than sodium.
As the hard water passes over the resin bed, the calcium and magnesium serve as stronger magnets. The resin beads instantly grab these hard minerals and, in the process, release the sodium ions into the water. This is the “exchange” in the hard water treatment process. The minerals that cause scale are trapped on the beads, and the water that exits the tank contains only the sodium ions, leaving it soft and safe for your plumbing.
Cation vs. Anion Exchange: Understanding the Difference
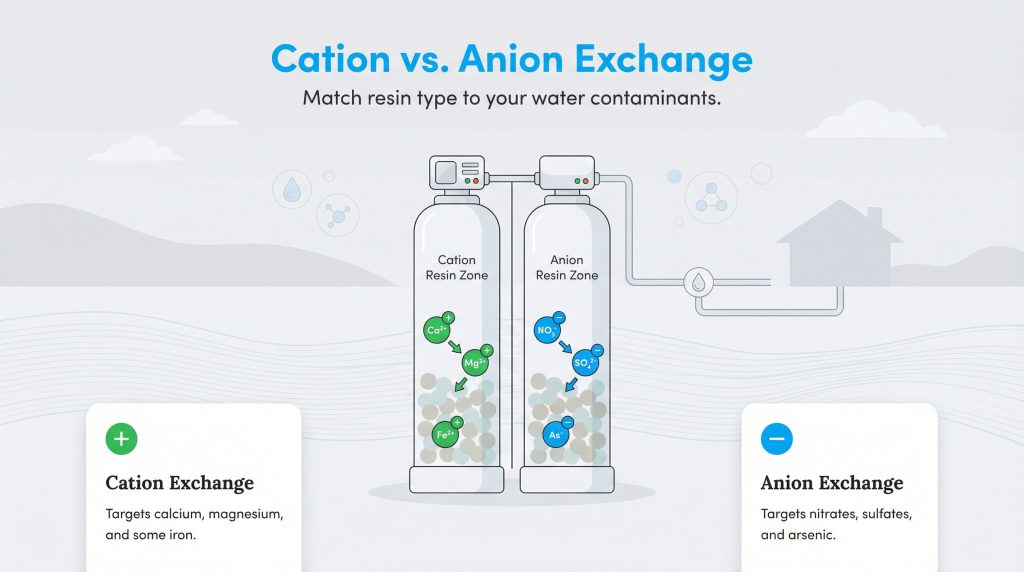
While water softening is the most common use for this technology, the type of resin used depends on what you need to remove. This brings us to the difference between cation vs anion exchange resin. It comes down to the electrical charge of the ions being targeted.
Cation Exchange resins are negatively charged and are used to attract positively charged ions. This is what you find in a standard water softener designed for water softening. It targets calcium, magnesium, and sometimes iron. Sometimes this is referred to as a deionization water system when used for high-purity applications, though residential softeners specifically target hardness minerals. On the other hand, Anion Exchange resins are positively charged and attract negatively charged ions. These are typically used for removing nitrates, sulfates, or arsenic from water supplies. Understanding which contaminants are in your water ensures you select the correct resin type for your home.
The Regeneration Cycle: Keeping Your System Running
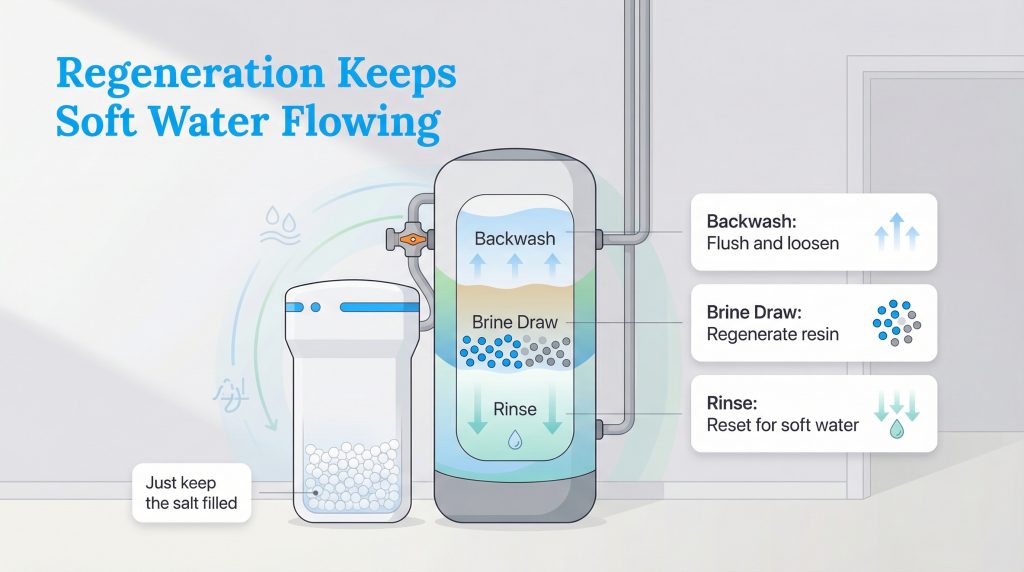
Eventually, those resin beads get “full” of hard water minerals and can no longer attract new ones. When this happens, the system must enter a water softener regeneration cycle to clean itself. This process uses a strong salt solution from the brine tank to wash the beads.
During the ion exchange resin regeneration process, the system floods the resin with brine. The sheer volume of sodium ions in the brine forces the calcium and magnesium off the beads and flushes them down the drain. Here is how the cycle generally flows:
- Backwash: Water flows backward through the tank to flush out dirt and loosen the resin bed.
- Recharge (Brine Draw): The salty brine solution is drawn into the mineral tank. The high concentration of sodium restores the positive charge to the beads, displacing the hard minerals.
- Rinse: Fresh water flows through the tank to remove excess brine, settling the resin bed back into place for service.
For you as the homeowner, this part is mostly automated. Your main job is simply to keep the brine tank filled with salt pellets so the system can do its work.
The Environmental Impact of Ion Exchange Systems

While effective, ion exchange systems do have an environmental footprint, primarily regarding water usage and sodium discharge. Older systems operated on a timer, regenerating every few days regardless of water use, which wasted salt and water. Modern systems use Demand-Initiated Regeneration (DIR), which monitors your water usage and only regenerates when necessary. This is a far more water efficiency focused approach that saves money and resources.
For those concerned about salt, there are salt-free conditioners available. However, it is important to note that these are not true softeners; they don’t remove hardness minerals but rather change how the minerals behave so they are less likely to stick to surfaces. If you want the removal benefits of ion exchange but want an eco-conscious alternative to standard salt, you have options. We also recommend checking your local municipal codes, as some areas have restrictions on salt-based softeners due to wastewater concerns.
Is an Ion Exchange System Right for Your Home?
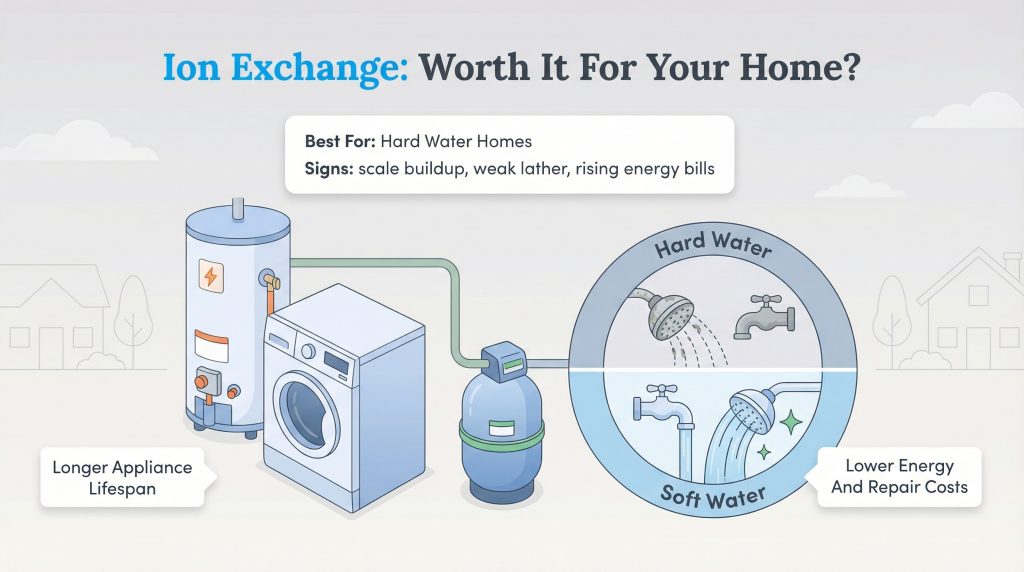
When you are deciding whether to install a system, you will end up balancing the advantages of ion exchange water treatment against the upfront cost. If your water hardness is above 7 grains per gallon (gpg) or you are seeing scale build up within weeks of cleaning, a softener is usually worth the investment. If you notice significant scale buildup on showerheads, your soap doesn’t lather well, or your water heating bills are creeping up, you are likely dealing with hard water. Scale buildup on heating elements acts as an insulator, which can increase energy use and shorten water heater lifespan.
The transition from hard water to soft water ion exchange can extend the lifespan of your washing machine, dishwasher, and water heater by years. However, it helps to weigh the pros and cons before committing:
| Pros | Trade-Offs |
|---|---|
| Extends the life of appliances | Requires regular salt purchases |
| Reduces energy costs for water heating | Discharges brine into wastewater |
| Cleaner dishes and softer laundry | Slight increase in sodium content |
While the initial investment includes the unit and installation, the long-term savings on appliance repairs and energy efficiency often pay for the system over time. You can learn more about how efficient appliances impact your utility bills in our guide on how to save on your electric bill. You can also explore more about how water quality affects your utility costs in our guide to residential water services.
Making Smart Water Decisions
Understanding the “how” behind your home’s utilities empowers you to make smarter maintenance decisions. An ion exchange system is a workhorse that operates quietly in the background, but knowing how it functions helps you troubleshoot issues and choose the right salt. Before you buy, we always recommend getting a simple water hardness test to ensure you size your system correctly for your specific needs.
Bringing It All Together For Your Home’s Water
Ion exchange is a proven, reliable way to banish hard water headaches from your home. By swapping troublesome calcium and magnesium for harmless sodium, these systems protect your investment in your home’s plumbing and appliances. Whether you choose a standard salt-based system or opt for potassium chloride, understanding the regeneration cycle and basic maintenance ensures your water stays soft for years to come. We encourage you to test your water today and take the first step toward a cleaner, more efficient home.
Learn more about Water Filtration for your Home
FAQs About Ion Exchange Systems
What is the difference between ion exchange and reverse osmosis?
How long does ion exchange resin last?
Does ion exchange remove bacteria?
Is ion exchange water safe to drink?
Can I install an ion exchange system myself?
About the Author
LaLeesha has a Masters degree in English and enjoys writing whenever she has the chance. She is passionate about gardening, reducing her carbon footprint, and protecting the environment.
